About the Event
The ISoP- Africa chapter meeting, will gather over 250 pharmacovigilance experts, regulators, and industry professionals to discuss “Opportunities and key challenges for enhancing patient safety in Africa“.
Advancing Pharmacovigilance Practice in Africa: Moving from Data collection to Data Driven Decision Making.
Thematic Areas
- Signal management and risk communication
- Regulatory harmonization for pharmacovigilance
- Digital health and patient engagement in pharmacovigilance
- Pharmacovigilance in Africa – a manufacturer’s perspective
- Impact of the African genetic background on adverse events and the future pharmacovigilance
- Active monitoring of vaccines and medicines
ISoP Africa Chapter 2024 Annual Meeting
Chairpersons
Deirdre McCarthy, Senior Program Officer, Integrated Development, Bill & Melinda Gates Foundation
Chairpersons
Chairpersons
David Nahamya, Secretary to the Authority
Dr Medard Bitekyerezo, Chairperson Board NDA
Symerre Grey- Johnson, Director Human capital and institutional development, AUDA_NEPAD
Dr Yonas Tegegn Woldemariam, Country Representative WH
Chairpersons:
Chairpersons:
TBC
Chairpersons:
Chairpersons:
Chairpersons:
PARTICIPATE IN OUR FIRST ISOP AFRICA CONFERENCE
Advancing Pharmacovigilance Practice in Africa: Moving from Data collection to Data Driven Decision Making.
Meet the SCIENTIFIC COMMITTEE
Meet the SPEAKERS
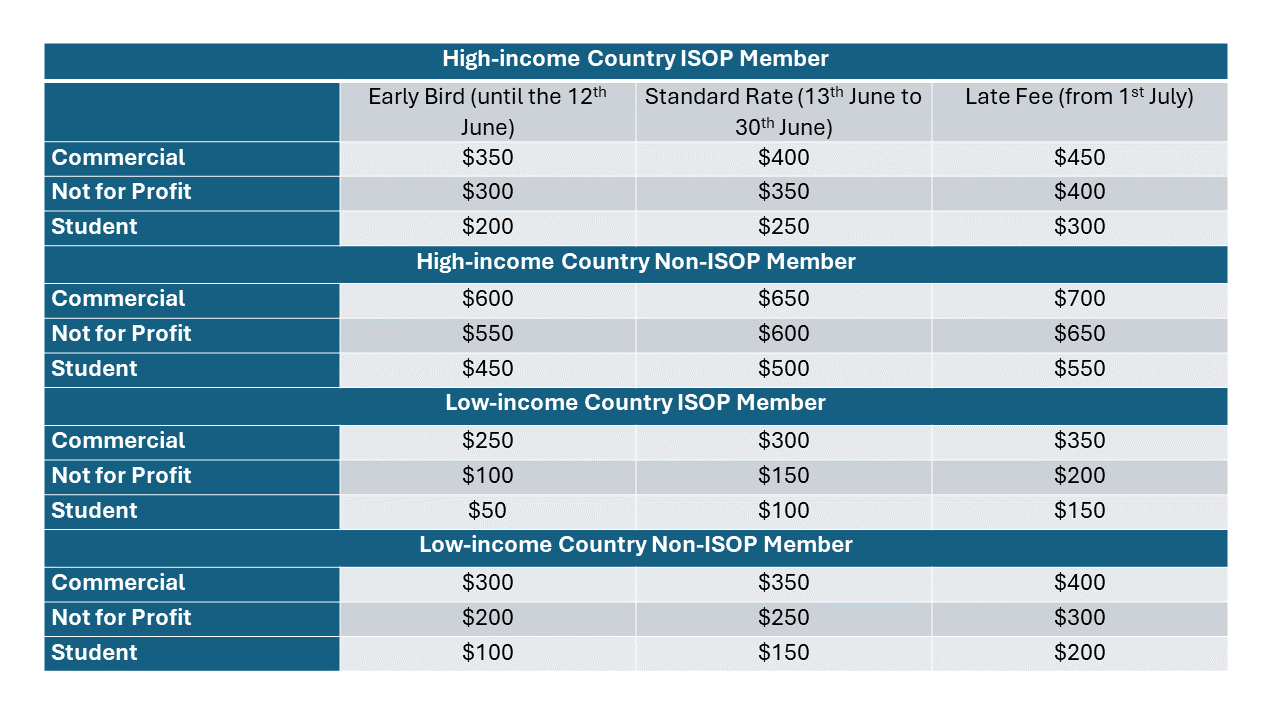
Registration FEES
ISoP Membership
- Members of ISoP are eligible for the “member” rate. If the ISoP Africa Chapter cannot verify your current membership, you will be charged the non-member rate.
- Non-member rates automatically include a one-year ISoP membership based on the selected fee category.
- Information to activate your account and enjoy all ISoP membership benefits will be sent with your final confirmation.
Hotel Accommodation
Please note that hotel accommodation is NOT included in the registration fee and must be booked separately. We have secured preferred rates at the conference venue and you can use this link below to make the bookings.
Also there are a number of hotels close to the conference venue.
IntuVigilance Africa Ltd
Event Management Organisation is IntuVigilance Africa Ltd who is acting on behalf of ISoP Africa Chapter
Registration Terms and Conditions
- Members of ISoP are eligible for the “member” rate. If the ISoP Africa Chapter cannot verify your current membership, you will be charged the non-member rate.
- Non-member rates automatically include a one-year ISoP membership based on the selected fee category.
- Information to activate your account and enjoy all ISoP membership benefits will be sent with your final confirmation.
Please note that there is a separate charge to attend the Gala Dinner – please indicate this when making a booking. There will be NO access to the dinner unless you have paid for this beforehand.
Please note that hotel accommodation is NOT included in the registration fee and must be booked separately. We have secured preferred rates at the conference venue and you can use the link below to make the bookings. Also listed are a number of hotels close to the conference venue.
Ugandan tourist visa costs $ 50. Tourist visa applications can be completed and paid for online at https://visas.immigration.go.ug/.
This event is being organised by The Africa Chapter of International Society of Pharmacovigilance (ISoP). By completing this registration form, you confirm and acknowledge that you are attending the event in your capacity as a healthcare professional or scientist.
In order to qualify for any ‘early bird’ rates, booking and direct payment received before the deadline date listed in the conference marketing material. Inclusive offers cannot be split between two people
All registrations must be completed (and paid) prior to attending the conference. Please note there will NO bookings taken on site.
Delegates may nominate an alternative person from their organisation to attend up to 48 hours prior to the start of the event, at no extra charge assuming that the substitute person holds the same membership status or additional fees may be required.
Should substitution not be possible, cancellation charges apply as follows:
- 8 weeks or more prior to start of event: 10% of the delegate fee
- 4 to 8 weeks prior to start of event: 50% of the delegate fee
- 4 weeks or less prior to start of event: 100% of the delegate fee
All substitutions and cancellations must be received in writing by email to secretariat@isopafrica.com
Our preferred method of payment is credit/debit card. We accept most credit/debit cards and you will have an option to pay via MTN mobile money, AIRTEL money or EZEE money.
It is the responsibility of the delegate to ensure payment is made. ISoP Africa Chapter will not chase sponsors or employers for payment on the delegate’s behalf.
Delegates will NOT be allowed to enter the event if the registration fee is not paid in full.
ISoP Africa Chapter reserves the right to postpone, alter and/or cancel all or any part of the event (including in-person day(s)) for any reason (including, without limitation, by reason of a force majeure).
In the event that all or any part of the event is postponed, altered or cancelled for any reason, delegates will be notified as soon as practicable prior to the advertised dates of the event. ISoP Africa Chapter accepts no liability in respect of any loss or damage, whether direct, consequential or otherwise arising from any alteration (including, without limitation, a change to the format of delivery of the event or a change of event venue), postponement (including, without limitation, any delay in providing the event), or cancellation of the event. ISoP Africa Chapter shall not be liable to delegates for any costs incurred in connection with accessing/attending the event.
ISoP Africa Chapter reserves the right to alter the programme and/or speakers without prior notice and without any liability to delegates. ISoP Africa Chapter does not accept responsibility for the views expressed by the individual speakers.
Please note that this event is kindly supported by a range of exhibitors and sponsors. ISoP Africa Chapter would like to thank the sponsors and all exhibitors for their support of the event. A full list of sponsors and exhibitors will be available on the event website. Sponsors and exhibitors have had no input into the arrangements for the event, including the agenda, content and speakers.
Photographs and video recordings may be taken and made at the conference by an official ISoP Africa Chapter photographer/ videographer. ISoP Africa Chapter may use these photos and videos in post-event publicity and on future marketing materials. In registering to attend the conference, delegates agree to the official photographer/videographer taking such photographs and videos, and to ISoP Africa Chapter using the photographs and videos as set out above. If you do not wish to be in any photography or video, you must notify a member of the ISoP Africa Chapter team immediately upon arrival at the conference for further guidance.
Delegates are not permitted to make their own video or audio recordings of any part of the event or use any photographs or videos for commercial purposes, unless they have ISoP Africa Chapter’s permission.
Please note: other attendees at the conference (delegates, speakers, exhibitors and sponsors etc.) may also take photographs and videos. ISoP Africa Chapter accepts no responsibility in connection with the use of these photographs or videos.
Delegates must not share any event content (including satellite symposia content) or access to such content with anyone else, in particular lay persons.
Delegates, including those sponsored or employed by pharmaceutical companies may not promote their sponsors and/or employers, or the products or services conducted by such sponsors and/or employers outside of their allocated exhibition stand (if relevant). This includes (without limitation) promotion by way of branded items, such as the wearing of branded clothing outside of the exhibition hall.
Canvassing for orders by delegates or any unauthorised person, including the display or distribution of materials, is strictly prohibited. Canvassing is only permitted by exhibitors (on their own stands), at sponsored satellite symposia (in agreement with ISoP Africa Chapter), via official promotion options and by authorised individuals.
ISoP Africa Chapter reserves the right, without any liability, to refuse a delegate access or eject them from the digital or in-person event for failure to comply with these Terms and Conditions; or if in ISoP Africa Chapter’s opinion a delegate represents a security risk, nuisance or annoyance to the running of the event. Delegates agree to follow all Covid-19 related rules and requirements imposed by ISoP Chapter Africa or the event’s venue, even where they go beyond Government guidelines.
ISoP Africa Chapter takes data protection and your privacy seriously. We will use the personal data you provide to us to:
Process your registration and provide applicable conference updates and services. We may need to share your data with third parties working for us, such as the conference venue and security providers. We will not provide or sell your personal data to other companies for marketing purposes without your agreement (see below for circumstances in which your information may be provided to sponsors or exhibitors).
ISoP Africa Chapter will not take responsibility for any personal information or data you choose to share with exhibitors or delegates directly at the event.